Difference between revisions of "Main Page/BPHS 4090/Optical Tweezers of Onions"
| Line 4: | Line 4: | ||
<h2> The Physics of Optical Tweezers </h2> | <h2> The Physics of Optical Tweezers </h2> | ||
| + | The principle of optic tweezers, first proposed and discovered by Arthur Ashkin in 1970.<ref> Ashkin, A. (1970). "Acceleration and Trapping of Particles by Radiation Pressure". Phys. Rev. Lett. 24: 156–9. doi:10.1103/PhysRevLett.24.156. http://prola.aps.org/abstract/PRL/v24/i4/p156_1.</ref> The principle relies on the refraction of light as it passes between media of differing indicies of refractions (symbolized by ''n''), and the Gaussian profile of a trapping laser beam which is most intense at the central axis. | ||
| + | |||
| + | |||
Revision as of 15:38, 22 July 2010
Introduction
The Physics of Optical Tweezers
The principle of optic tweezers, first proposed and discovered by Arthur Ashkin in 1970.[1] The principle relies on the refraction of light as it passes between media of differing indicies of refractions (symbolized by n), and the Gaussian profile of a trapping laser beam which is most intense at the central axis.
Structure of an Onion Cell
Plant cells have the general properties of a rigid cell wall, a large open vacuole, a nucleus, and cytoplasm containing organelles in the spaces between the cell walls and vacuole. The onion cell is a classic and often-used example of this structure. The organelles in the cytoplasm are small (between 0.5 and 1 micron), and roughly spherical in nature- prime candidates for optical tweezing with a laser. Organelles move throughout the cyctoplasm
Preparation of onion epidermis for optical trapping experiments [3]
The common onion (Allium cepa) is often used to examine individual plant cells because of the ease of isolating sheets of cells that are one cell thick. The onion bulb can be sectioned into quarters or eighths. Then, the individual scale leaves can be separated.
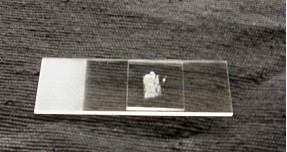
The exposed concave surface can be scored with a sharp razor blade. With a very fine pair of forceps, pieces of the epidermis can be lifted (note the transparency of the peel). Before doing so, have a microscope slide ready with a drop of distilled water (or artificial pond water) so that the peel doesn’t become dehydrated. Unlike the photographs, a thin strip of Vaseline will be placed on the microscope slide in a rectangular shape slightly smaller in dimensions then the cover slip. Having placed the epidermal peel in the water inside the Vaseline ‘dike’, carefully (gently) place the coverslip on top, pressing to create a seal around the perimeter.
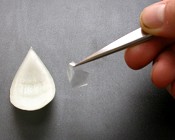 File:Onion5.jpg
File:Onion6.jpg
File:Onion5.jpg
File:Onion6.jpg
Now ready to place in the holder on the optical bench (left), here is what the cells will look like (right, the nucleus and transvacuolar strands are indicated).

To view an animation on how the myosin motors move the spherosomes along the actin fibers: [[1]]
Lab Exercise: Optical tweezing of onion cells
Apparatus
Computer Control
Calibrate Optical Trap Depth
Determine Strength of Myosin Motor
References
- ↑ Ashkin, A. (1970). "Acceleration and Trapping of Particles by Radiation Pressure". Phys. Rev. Lett. 24: 156–9. doi:10.1103/PhysRevLett.24.156. http://prola.aps.org/abstract/PRL/v24/i4/p156_1.
- ↑ Source: N. S. Allen and D. T. Brown, 1988. Dynamics of the Endoplasmic Reticulum in living onion epidermal cells in relation to microtubules, microfilaments, and intracellular particle movement. Cell Motility and the Cytoskeleton 10:153-163
- ↑ Diagrams from Peterson LR, CA Peterson, and LH Melville (2008) Teaching Plant Anatomy through Creative Laboratory Exercises. National Research Council of Canada. Page 17.


