Difference between revisions of "Main Page/BPHS 4090/Lysozyme Crystallization"
| Line 119: | Line 119: | ||
</p> | </p> | ||
</td></table> | </td></table> | ||
| − | + | <br style="clear: both" /> | |
<table width=300 align=center><td> | <table width=300 align=center><td> | ||
Revision as of 12:53, 2 September 2010
Contents
Introduction
Three-dimensional Protein Structures
X-Ray Crystallography
What is a Protein Crystal?
Suggested Reading:
Introduction to Macromolecular X-Ray Crystallography by Esko Oksanen and Adrian Goldman (Chapter 10 in Comprehensive Natural Products II Chemistry and Biology, Editors-in-Chief: Lew Mander and Hung-Wen (Ben) Liu ISBN: 978-0-08- 045382-8).
Alexander McPherson. Introduction to protein crystallization. Methods, 34, 254-265.
Protein X-Ray Crystallography Methods by Roger S. Rowlett, Department of Chemistry, Colgate University
Websites
- http://www.ruppweb.org/level1/new_tutorials_page.htm
- http://www.sdsc.edu/Xtal/edu.index.html
- http://www.hamptonresearch.com
- http://www.pxuniverse.com/
Experimental
You will be provided with the pure protein (lysozyme) that you will be crystallizing. You will also be provided with the crystallization solution. In real life you perform crystallization trials with your homogeneously pure protein and examine your plates looking for protein crystals.
Crystallization Experiment I
Stock Solutions
Protein
Lysozyme at 25, 50, 75 and 100 mg/ml dissolved in 0.02 M sodium acetate pH 4.6
Lysozyme Crystallization Solution
- 0.6 M sodium chloride
- 0.1 M sodium acetate pH 4.6
- 25% Glycerol
Procedure
|
Figure 1: Crystallization Plate
|
- Fill reservoirs with 500 μl of lysozyme crystallization solution.
- Add 1 μl of the crystallization solution to 1 μl of lysozyme on the cover.
- Repeat for the remaining lysozyme concentrations. You should have 4 drops on each cover. Keep in mind where you place the different lysozyme concentrations by referring to the notch.
- Screw cover on reservoir.
- Repeat for the remaining reservoirs in your row.
- Incubate at room temperature.
- Observe and document conditions in drops with microscope immediately after setup and 1, 2 and 3 weeks later.
|
Figure 2
|
Describe the results! Do you see any trends? How many crystals do you see per drop? Which drop contains the largest crystals? Which drop contains the most crystals?
Describe Vapor Diffusion as it pertains to crystal growth.
|
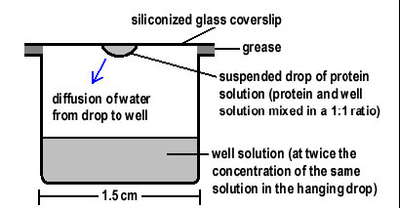
Figure 3: Well setup for crystal growth by the vapour diffusion method
|
Crystallization Experiment II
Stock Solutions
Protien
Lysozyme at 25, 50, 75 and 100 mg/ml dissolved in 0.02 M sodium acetate pH 4.6
Crystallization Conditions
Qiagen JCSG+ Screen
This crystallization screen contains 96 known solutions. You will use 24 of these conditions to setup an initial crystallization screen for the 4 different lysozyme concentrations.
Prepare the crystallization plate as above but remember to use a different crystallization condition in each well. The crystallization conditions are labeled 1-24. Each group will set up 6 different conditions in one row.
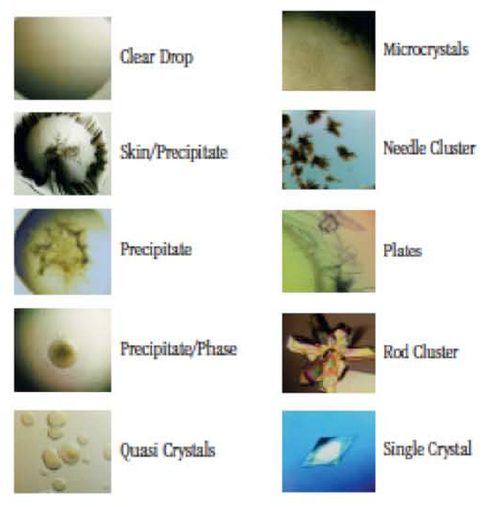
Observe and document the drops with microscope. Shown below are typical drop results. Most drops will be either clear (no crystals, no precipitate) or contain precipitate (protein).
Describe the results! Do you see any trends? How many drops are clear? How many drops have precipitate? Do any drops contain crystals?
|
|
Protien Crystal Mounting
Many protein crystals are extremely sensitive to changes in temperature and surrounding conditions (i.e., presence of original solvent). As well, the protein crystals 5 undergo radiation damage upon exposure to X-rays. Therefore, the crystal must be flash-cooled to 100K in a stream of liquid nitrogen to prevent damage from the highintensity x-rays. Cryoprotectants such as glycerol are used to prevent freezing of the water surrounding the protein crystal which may destroy the protein crystal.
|
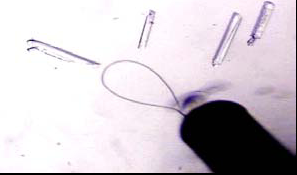
Figure 5: Different pins containing a loop on the top end.
|
|
Figure 6: Using a pin to mount a crystal in a loop.
|
|
Figure 7: Using a pin to mount a crystal in a loop observed through
the microscope. The size of the crystal dictates the size of the loop. The crystal will be
scooped into the loop and mounted on the goniometer head for diffraction analysis.
|
You will be given an opportunity to mount the lysozyme crystals into the loops during the lab.
|
Figure 8: Showing the pin containing the protein crystal mounted on the goniometer head
on the diffractometer.
|
You will test the diffraction potential of your lysozyme crystals using a Rigaku 007HF Microfocus X-ray machine equipped with a Saturn 944+ CCD.
Describe the results. What is the diffraction resolution of your lysozyme crystals? What is the space group?