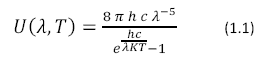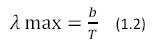Difference between revisions of "Main Page/BPHS 4090/Choloplast Translocation"
| Line 47: | Line 47: | ||
photosynthetic pigments, principally chlorophyll. The absorption spectrum of chlorophyll a is shown in | photosynthetic pigments, principally chlorophyll. The absorption spectrum of chlorophyll a is shown in | ||
Figure 1 <ref>http://www.bio.davidson.edu/courses/Bio111/Bio111LabMan/lab1fig3.gif</ref>.</p> | Figure 1 <ref>http://www.bio.davidson.edu/courses/Bio111/Bio111LabMan/lab1fig3.gif</ref>.</p> | ||
| + | |||
| + | |||
| + | <References/> | ||
Revision as of 12:51, 15 September 2010
Required Components
- Stock culture of Eremosphaera viridis
- Glass slides, cover slips and micropipette
- Nikon Optiphot Microscope
- Radiometric Probe (Model S471 Portable Optometer, UDT Instruments)
- Cool Snap CCD Camera and MicroManager software
- Blue and red band-pass filters
Objective
To observe the wavelength dependence of light on chloroplast translocation in the acidophile green algae Eremosphaera viridis as well as describing a mechanism behind this response.
Introduction
Electromagnetic radiation is the driving force for photosynthesis. Plants and other autotrophs convert the energy of light into chemical energy used for synthesizing carbohydrates and other organic compounds, which heterotrophic organisms use for energy and other nutritional requirements. Photosynthesis is a unique process performed by plants, algae, and some species of bacteria. It consists of a series of oxidation and reduction reactions; the basic chemical formula is shown below:
|
|
Photosynthesis would not be possible without the energy derived from light. Electromagnetic radiation enters the earth’s atmosphere from the sun where it is harvested by photosynthetic organisms. Wavelengths between 400 and 800 nm are used by photosynthetic organisms. The sun can be approximated as a near perfect blackbody with a surface temperature of 5800 K described by Planck’s Black Body Radiation Law, shown in Equation 1.1
|
|
Where U (λ, T) has units of J m-2 s-1, h is Planck’s constant, K is Boltzmann’s Constant and c is the speed of light. The color temperature of the sun corresponds to a peak wavelength emission of about 500nm according to Wien’s Displacement Law (Equation 1.2)
|
|
Where b = 2.898 x 10-3 in units of m*K.
The first step in the conversion of the energy of photons to chemical energy is absorption by photosynthetic pigments, principally chlorophyll. The absorption spectrum of chlorophyll a is shown in Figure 1 [1].