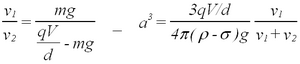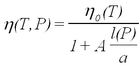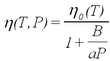Main Page/PHYS 3220/Millikan
Contents
Determination of the Electric Charge Unit: The Millikan Oil-Drop Experiment
Abstract
In this experiment charged droplets of oil, in air, moving under gravitational and electric fields are observed to verify the quantization of charge. The electric charge unit is determined to a few-percent accuracy. From the Brownian motion of the oil droplets one also obtains an estimate of Boltzmann’s constant.
Introduction
Millikan's determination of e, the charge of the electron (1909), proved that electric charges are quantized, i.e., occur as integer multiples of a unit. The measurement is performed on oil droplets that are ionized by the process of vaporization. To the present day it is true that for all elementary particles that can be freely observed, their electric charge is given as an integer multiple of a unit, i.e., q = n e. There exist, however, confined particles (quarks) that carry fractional charges of 1/3 and 2/3 of e.
The experiment makes use of an atomizer that generates oil droplets, some small fraction of which acquire positive or negative charges during the atomizing process. An electric condenser that can be charged to several hundreds of volts, and an illumination device as well as a telescope are included to observe the motion of the oil droplets. (Note that a radioactive source was used by Millikan to change the charge of droplets).
Under gravity the droplets move downwards. However, they are not in free-fall as a result of the viscosity of air, and the forces due to viscosity is described by Stokes' law. This frictional force results in a constant terminal velocity. Knowledge of the density and viscosity allows us to determine the mass of the droplet from the measured terminal velocity. The size of the droplet can be calculated by knowing the density of the oil.
Using an electric field of the right direction and magnitude Vh it is possible to counterbalance the gravitational force, i.e., to suspend the droplet (viscosity is irrelevant for a stationary droplet). This permits us to determine the charge of the droplet after the mass has been determined in the previous step.
An alternative method involves a measurement of the terminal velocity for a fixed voltage V that counteracts gravity. This was used by Millikan who did not have a continuously adjustable power supply to dial up the correct voltage for any given droplet, i.e. Vh. Both methods are used independently in this experiment.
A correction (due to Millikan) for the fact that the viscosity becomes smaller for droplets of a size comparable to the mean free path of air molecules has to be introduced for an accurate determination of the charge unit.
Theory
A charge q, situated in an electric field E experiences a force F = q E. If a potential difference V is applied to two parallel plates separated by a distance d, the constant electric field between the plates is perpendicular to the plane defined by the plates and its magnitude is given as E=V/d. Thus, by changing the voltage it is possible to counteract the gravitational acceleration g and hence to suspend a macroscopic particle of mass m with charge q according to
| (1) |
To determine q it is necessary to know m, i.e., a measurement of the voltage that suspends the particle Vh is not sufficient to find the value of q. It is possible to find the mass m from a determination of the motion of the object without electric fields applied. However, it is necessary to take into account the viscosity of air at the given temperature and pressure thermometer and barometer (Digital). For gases (and fluids) a simple model of the viscous force is provided by Stokes' law. According to this law freely falling bodies reach a force equilibrium over a short distance with a terminal velocity v1. For a spherical droplet of oil with radius a and a given viscosity η for the medium through which the droplet moves, the frictional force is given by
| (2) |
Here we take into account the density of air σ as well as the density of oil ρ to determine the gravitational force (buoyancy) as
| (3) |
Thus, a determination of the terminal velocity v1 that arises from the condition FS=Fg allows us to find the radius of the droplet (and thus the mass). Since the radius of the droplet is relevant for a refined discussion of the viscous force, it is worthwhile to calculate it for each observed droplet from the terminal velocity v1 under gravity (derive!):
| (4) |
This estimate is based on the assumption of Stokes’ law, and, thus, in his later work Millikan has also used an expression that makes use of both terminal velocities (ref. 1). It requires a previous estimate of the electric charge unit e, and is obtained from a division of the conditions of force equilibrium with and without an electric field (derive!):
| (5) |
Here v2 is the magnitude of the velocity obtained for the condition when a voltage V is used to overcome gravity and make the droplet move towards the upper plate of the condenser. In the limit of v2 = 0 (suspended droplet) an estimate of the droplet radius can be made from the holding voltage Vh itself. While this procedure may seem unsatisfactory, since it depends on a knowledge of the charge value e (or a multiple thereof for a multipli-charged droplet, q=ne), a discrepancy between the determination of the charge radius from eqs. (4) and (5) points clearly to a problem with the knowledge of the viscosity for droplets of varying size.
A small modification of the force balance is required if an arbitrary voltage is applied. In Millikan's original setup only fixed voltages V could be obtained from a battery, and therefore, it was necessary to determine two terminal velocities: v1 for V=0, and v2for the case where the voltage overcomes gravity. Derive the expressions required to calculate the charge of the droplet for both methods. The buoyancy of air is so small compared to the gravitational pull on the droplet that it can be neglected ( σ << ρ ).
The charge of the droplet when using method (I), i.e., finding Vh and the 'free' terminal velocity v1 is given as (using MKSA units, an approximation is given for quick lab calculations (use v1 in m/s):
| (6) |
For method (II) the charge is obtained from the expression
| (7) |
A remaining problem concerns the applicability of Stokes' law for small droplets. Viscosity is caused by intermolecular forces in the medium (in our case the air). The Stokes law describes a macroscopic force that averages over the Brownian motion of molecules. Kinetic theory is capable of calculating values of viscosity for matter on the basis of modeling molecular collisions. The validity of the force law becomes questionable for particles whose size is comparable to the mean free path l of the air molecules. After several years of study Millikan reported the results of systematic investigations of oil drop charge measurements as a function of atmospheric pressure in the chamber (i.e., variation of l). The correction of the viscosity is expressed in terms of the dimensionless parameter l/a as (ref. 2):
| (8) |
Here η0(T) is the temperature-dependent viscosity at room pressure, l(P) is the pressure-dependent mean free path, and A is a constant determined experimentally by Millikan (ref. 1). While the variation of the viscosity with temperature is not too significant (see Fig. 1.3 in ref. 2), the overall correction from eq. (8) for droplets of typical size, of a = 0.7 μm results in a reduction of the charge value by about 15%. Note that the viscosity enters into the expression for the charge (eq. 6) with the power of (3/2)! It is convenient to express eq. (8) in terms of air pressure:
| (9) |
Are inter-droplet forces of importance when you inject a small cloud of oil droplets into the chamber?
In this expression the pressure P is expressed in cm of Hg, the radius of the oil drop a is in cm, and the empirical constant B is given as 6.17 x 10-4.
While observing slow droplets (small ones under free fall or larger ones for voltages near Vh) one notices a large deviation in the fall times, i.e., the standard deviation σt2 stabilizes rather than going to zero as the number of observations is increased. This is a direct manifestation of the jitter from the Brownian motion of the droplets. The droplets acquire this motion from collisions with the surrounding air molecules. For not too short average fall times τ2 (that are used to estimate the terminal velocity) one can assume that the standard deviation of the times from the average is entirely due to the Brownian motion. The deviation σt2 should increase then with the average time as τ2 . The dimensionless coefficient that relates these two quantities should decrease with increasing viscosity of the medium and increase with the thermal energy of the medium. Thus, qualitatively we can understand that it is given by the ratio of thermal energy to viscous energy (force x total path travelled = s, and v=s/τ). For a derivation see ref. 3, and for further discussions of the measurement problems see ref. 4,5. We can determine the Boltzmann’s constant kB from
| (10) |
using the standard deviation from repeated fall or rise time measurements and the room temperature in degrees Kelvin.
Summarizing, we find that the analysis of the oil drop experiment allows us to measure indirectly the size of a tiny sphere, determine its mass (verify in kg for your droplets), and observe ionized droplets where one of very many (how many for your droplet?) electrons are removed. In addition we obtain a direct observation of thermal (Brownian) motion and gain insight into the mechanisms responsible for viscosity.
Experimental Procedure
WARNING: There is a potential hazard of electric shock in this experiment. Be careful not to disconnect the cables to the condenser plates while voltage is applied.
The apparatus consists of an oil vaporizer, a high-precision parallel plate condenser, an illumination lamp with thermal shielding to prevent heat convection currents inside the chamber, and a telescope with a calibrated scale (recticule) and a very narrow focal range. A power supply provides the variable voltage to the condenser plates (shock hazard!) and also supplies the lamp. A CCD camera is interchangable with an eye piece allowing the drops to be viewed either on the computer (easier!) or directly through the eye piece.
Familiarize yourself with the equipment. The experiment requires patience and accurate observations. Note that while adjusting the focus of the telescope by moving it forward the chamber may be disturbed, and this should be avoided (especially during observation). The chamber has to be aligned vertically in the gravitational field, and air drafts should be kept to a minimum to avoid horizontal drifting of droplets. Inspect the vaporizer set-up for the injection of droplets.
Trace the high-voltage connections and recognize for which charge sign the droplets will be attracted to the upper condenser plate by the electric field. Turn on the power supply, set the voltage to about 500 V. Align the nozzle of the oil vaporizer with the holes in the chamber, and squeeze the ball firmly (once). Observe how a cloud of droplets becomes visible in the field of view due to illumination by the lamp. Particles at a well-defined distance can be brought into focus. The distance measurement with the recticule works only if the droplet is in focus. Measure the scale conversion to actual distance, by observing the grading on a ruler. The ruler should be placed where the oil droplets are observed after the chamber has been removed (first turn off the high-voltage !).
It is possible to swing the telescope (carefully) somewhat sideways to align the scale with the vertically moving droplets (do not jerk the apparatus). Turn the voltage off and on to observe which droplets react to the electric field in the desired way. Note that the telescope does not contain an image inverter lens, i.e., real downward motion appears as upward motion in the field of view.
Learn how to trap particles in the lower half of the field of view (the upper half is likely to be obscured at least initially). Repeated measurements with a stop watch of the times for field-free fall, and the rise times under field, are required to estimate the errors incurred. Be aware of potential parallax errors. Voltages required to suspend the droplet should be carefully noted. Note that under holding conditions a jittery (thermal) motion of the droplets becomes visible. If a bright droplet becomes dim after some time, it has drifted out of the volume illuminated by the lamp. If you lose a droplet, search for other bright ones: the pattern of switching the voltage on and off prevents many droplets of similar mass and charge from hitting one of the condenser plates.
Using the CCD Camera
The AmScope MD800E CCD camera is attached to the computer using a usb cable. The software used to operate the camera is called Amscope. After running the program, click on the icon on the top toolbar that is for "Live Capture" and select the device "AmScope MD800E". A window will appear showing the real-image from the camera. You will have to calibrate distance to pixel size for our set-up. To do this, put something into the field of view of the camera of a known size, be sure you are in focus, and then determine the number of pixels which correspond to the known thickness. You may find the "measure" tool of the AmScope software useful for this.
You can record data to a video for analysis later on by using the Start Capture Video found in the Capture menu.
To obtain data for analysis follow these steps for 5 positive and 5 negative droplets:
- Suspend the droplet and note Vh using a digital voltmeter.
- Record repeated measurements of terminal velocities v1(V=0) and v2(e.g., at Vmax=585 V) using a stopwatch while observing how the droplet passes, e.g., 20 scale marks (approx. 1 mm). Try to repeat for each droplet the fall-rise cycle at least 10 times. This can reveal systematic deviations: if a droplet drifts away from (or towards) the telescope the conversion of scale marks to actual distance may change. You may be lucky and observe a charge exchange reaction between the droplet and an air molecule, i.e., the droplet may lose or increase its charge. In the latter case try to continue taking measurements - you will obtain a remarkable proof of charge quantization. Attempt to select droplets of different sizes. This can be achieved by looking for droplets suspended by different voltages Vh.
- Assemble your data in a table. Calculate charge values using {Vh, v1} and {v1, v2} respectively. How consistent are the charge assessments from both methods? Calculate the radius of each droplet using both eqs. (4) and (5). Calculate corrected charge values based on the correction of the viscosity given in eq. (9).
- Form the average of the corrected charge unit with standard deviation and compare with the value of e = 1.602 x 10-19 As.
- (Optional) If your droplets can be grouped approximately in two groups according to size, calculate the average with standard deviation for the uncorrected charge values for each group. Do you have enough significant information to observe that the smaller droplets carry a larger apparent (uncorrected) charge? Does this information confirm the validity of the ideas behind eq. (9)?
- Estimate Boltzmann's constant using eq. (10) from those droplets for which many rise and fall times were recorded. Compare with kB = 1.38 x 10-23 J/ K .
Give careful consideration to errors and their analysis. It is advantageous to calculate charge values based on each pair of subsequent fall and rise times, and to average the final result. This eliminates potential errors due to evaporation of droplets as a function of time. Also note that the droplet-size dependent correction of the viscosity is typically different by 1-2 % depending on the method used to estimate the droplet size. If eq. (4) is used, an iteration is required in which the droplet radius is recalculated using the improved viscosity η(T, P) (cf.. ref. 4,5). Using a computer (in Maple or some programming language) will make the data analysis easier.
References
- Millikan, R.A., Phys. Rev. (Ser. I) 32, 349-397 (1911) and (Ser. II) 2, 109-143 (1913).
- Melissinos, A.C., Experiments in Modern Physics, Academic Press, pp. 2-8.
- Millikan, R.A., The Electron, University of Chicago 1917, chapter VII, and appendix C.
- Wall, C.N. and Christensen F.E., Am. J. Phys. 43, 408-13 (1975).
- Jones R.C., Am. J. Phys. 63, 970-77 (1995).
Appendix
Constants:
- At 18o C, ηair = 1.81 x 10-5 Newton-sec/m2
- 1 poise is the c.g.s. unit of viscosity and equals 1 dyne-sec/cm2
- ρ = 0.882 gm/cc = 882 kg/m3 (Leybold lists 0.875 gm/cc)
- g = 981 cm/sec2 = 9.81 m/sec2
- d = 0.6 cm = 0.006 m
- σ = 1.29 x 10-3 gm/cc = 1.29 kg/m3. It is normally neglected since it is very small compared to ρ.
A note on units:
V is measured in Volts; this is an MKSA unit. Thus in the equations v must be in m/sec, g in m/sec2, ρ in kg/m3, d in m, etc. and the charge q will be expressed in Coulombs.
If on the other hand, in these equations c.g.s. units are used, i.e., v in cm/sec, ρ in g/cc, d in cm etc. , the appropriate c.g.s. units of potential must be used. This is the "electrostatic unit of potential" and 1 Volt = 1/300 e.s.u. of potential. In this case q will be in e.s.u. of charge and 1 e.s.u. of charge = 1/3 x 10-9 C (1 C = 1 As).