Main Page/BPHS 4090/Mapping a binding site using NMR spectroscopy
In this experiment, you will be using studying a fragment of a protein called CASKIN. It is involved in neuronal signaling. When a neuron is stimulated, chemical messengers are transferred between two neurons at a specialized cell-cell junction called a synapse. CASKIN is a scaffolding molecule found at the pre-synaptic side of the connection. A scaffolding molecule, much like its name, helps assemble other protein together in a certain part of the cell.
In the box below, draw a schematic of a synapse and the approximate location where CASKIN.
|
|
CASKIN is composed of many protein domains, each with its own function. Not suprisingly, since CASKIN’s job in the cell is to bring together other proteins, it is composed exclusively of domains that promote protein-protein interactions.
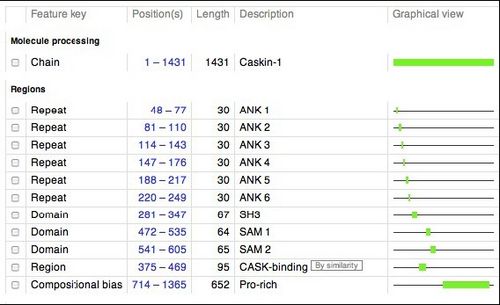
Below is a schematic of human CASKIN (1365 amino acids). This schematic was retrieved from the UNIPROT website (http://www.uniprot.org/uniprot/Q8WXD9), a respository of information on thousands of proteins. We will be studying the SH3 domain of CASKIN, found between amino acids 281 and 347 in the protein.
Members of the Donaldson laboratory used a technique called PCR to amplify the gene fragments encoding the SH3 domain, the first SAM domain, the second SAM domain, and the two SAM domains together. These fragments were inserted in to an expression vector, a circular piece of DNA or plasmid, that can be introduced into the common laboratory bacterium, Escherichia coli. Upon addition of a special kind of sugar into the culture which the bacteria cannot metabolize, the bacteria will begin to produce the human protein that we have programmed into them.
The cells were harvested and then cracked open. From the thousands of proteins in the bacterium the CASKIN SH3 domain was purfied in about two hours because it was tagged with a special sequence of six histidines that no other native bacterial protein had. Six histidines bind nickel very well so we used a special type of chromatography when nickel ions are exposed on a resin.
The protein was further purified by a technique called gel filtration that separated molecules according to their size. At this point the protein was extremely pure. It was then concentrated to about 10 mg /mL which doesn’t sound like much but it is !
From the NMR laboratory tour that you participated in, fill in the vital statistics of the CASKIN SH3 domain NMR sample:
|
length of NMR tube | |
|
width of NMR tube | |
|
volume range of NMR sample (mL) | |
|
ideal concentration of a protein [mM] | |
|
temperature range of experiments | |
|
temperature of the magnet (K) | |
|
field strength of the magnet (Tesla) | |
|
precession frequency of a 1H nucleus | |
|
precession frequency of a 15N nucleus |
The protein that was used to acquire the NMR data was uniformly isotropically labeled with 15N. To achieve this, the bacteria were grown in a medium containing 15N-ammonium chloride as their only source of nitrogen.
Why is 15N useful for NMR spectroscopy and not 14N, the natural abundance isotope ?
|
|
One of the first NMR experiments that is acquired on a protein sample is called a 2D 15N-edited HSQC. This experiment consists of a pulse sequence that selects only for 1H bound by one bond to a 15N. All other bonds such as 1H-12C and 1H-13C are not observed. An HSQC experiment is an extremely useful tool to gain and immediate understanding of the protein structure, it’s like a fingerprint of sorts. Here is the basic chemical formula of a protein. As you can see, there is an amide NH in the protein backbone. Thus, you would expect to see one NH resonance (or spot/peak) per amino acid. The CASKIN SH3 domain that we will be studying has the following sequence:
The quaternary amino group (NH3+) at the beginning of a protein is not visible by NMR methods. The 1H exchange with protons from water very quickly.
Some amino acids have NH in their side chains and other amino acids do not have any NH at all. Here is the sequence of the CASKIN SH3 domain exactly how it was made for the NMR experiments.